 Other Ingredients: Microcrystalline Cellulose, Sodium Starch Glycolate, Magnesium Stearate, Polyvinyl Alcohol, FD&C Yellow #6 Aluminum Lake, FD&C Blue #2 Aluminum Lake, FD&C Red #40 Aluminum Lake, Talc, Titanium Dioxide, and Propylene Glycol Other Ingredients: Microcrystalline Cellulose, Sodium Starch Glycolate, Magnesium Stearate, Polyvinyl Alcohol, FD&C Yellow #6 Aluminum Lake, FD&C Blue #2 Aluminum Lake, FD&C Red #40 Aluminum Lake, Talc, Titanium Dioxide, and Propylene Glycol Buy Direct » | NDC 52747-0307-30 | | 30 | | Tablets | | OTC | | | NDC 52747-0307-70 | | 100 | | Tablets | | OTC | |
| WARNING: Accidental overdose of iron-containing products is a leading cause of fatal poisoning in children under 6. Keep this product out of reach of children. In case of accidental overdose, call a doctor or poison control center immediately. | |
You should contact your healthcare provider for medical advice about adverse events. To report a serious adverse event, contact US Pharmaceutical Corporation, P.O. Box 360465, Decatur, GA 30036.
INDICATIONS: A hematinic supplement for iron deficiency anemia.
SIDE EFFECTS: Gastrointestinal disturbance (anorexia, nausea, diarrhea, constipation) occur occasionally but are usually mild and subside with continuation of therapy.
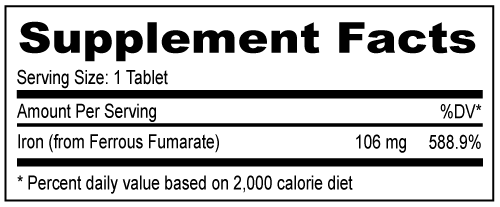
DOSAGE: Adults (persons over 12 years of age), one (1) tablet daily, between meals, or as prescribed by a physician. Do not exceed recommended dosage. Do not administer to children under the age of 12.
HOW SUPPLIED: Hemocyte® is a round, dark red film-coated tablet debossed with the "US" logo on one side. Hemocyte® is supplied in boxes of 30 tablets, NDC 52747-307-30 and 100 tablets, NDC 52747-307-70.
If you are pregnant or nursing a baby, seek the advice of a health professional before using the product. Do not purchase if any portion of product is opened. Store at controlled room temperature 15° to 30°C (59° to 86° F). Keep in a cool, dry place. Capsules are not USP.
Marketed by US Pharmaceutical Corporation, P.O. Box 360465, Decatur, GA 30036
Rev. 02/2015
|